▼▼▼▼▼▼▼▼▼▼▼▼
|
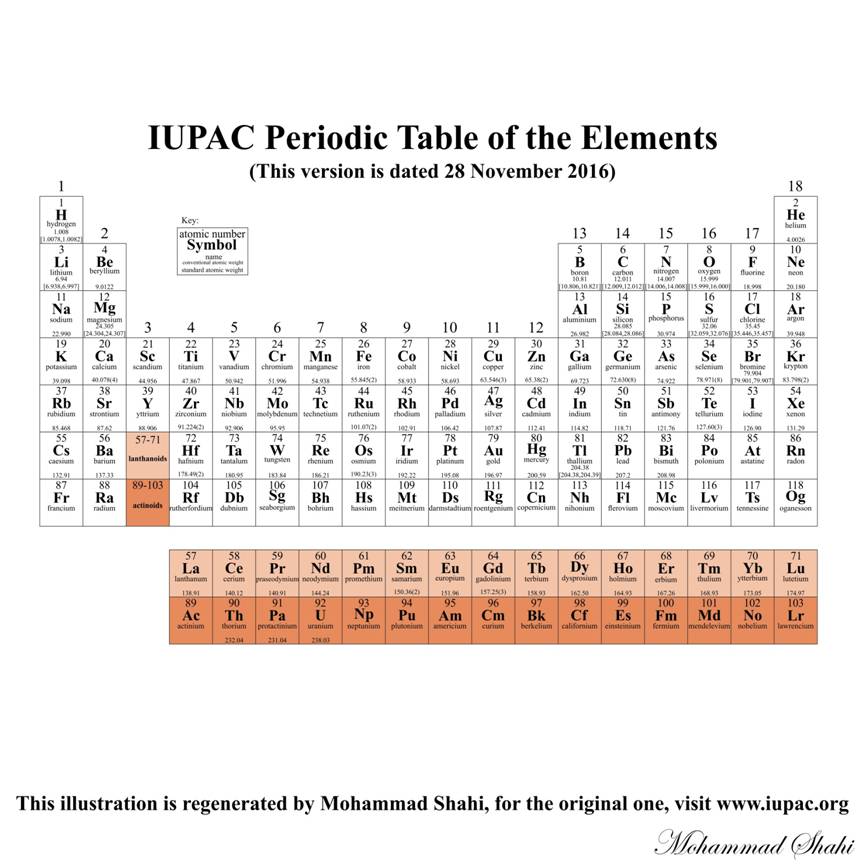
IUPAC Periodic Table of the Elements (2016)
The latest version of IUPAC Periodic Table of the elements currently available at www.iupac.org
The periodic table or the periodic table of the elements is a tabular arrangement of chemical elements listed according to increasing atomic number from left to right in horizontal rows called periods and from top to bottom in vertical columns called groups where the basis of the periodic table and its arrangement is the electronic configurations of the elements.
Russian chemist Dmitri Mendeleev was the first to publish a recognizable periodic table in 1869, developed mainly to illustrate periodic trends of the elements known up to then. Mendeleev's idea has been slowly expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior. The modern periodic table now provides a useful framework for analyzing chemical elements in many aspects, and continues to be widely used in chemistry, nuclear physics and other sciences.
The latest release of the Periodic Table by IUPAC (dated 28 November 2016) includes the recently added elements 113, 115, 117, and 118 with their names and symbols, the standard atomic weights 2013 (abridged to five significant digits) and the conventional atomic weights as published in PAC Vol. 88, No.3, pp. 265-291, and for ytterbium, the standard atomic weight updated following the 2015 review. An interval in square brackets provides the lower and upper bounds of the standard atomic weight for that element. For users needing an atomic-weight value for an unspecified sample with disregard to the uncertainty, the conventional values are provided. No values are listed for elements which lack isotopes with a characteristic isotopic abundance in natural terrestrial samples.
(for more information see https://en.wikipedia.org/wiki/Periodic_table)
|


