▼▼▼▼▼▼▼▼▼▼▼▼
|
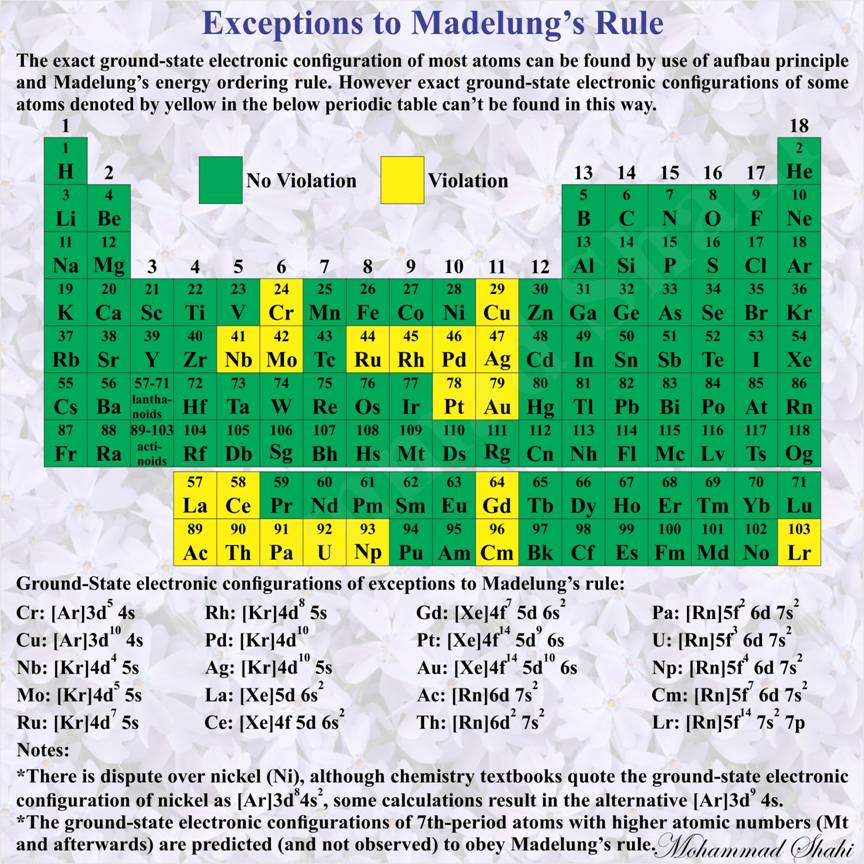
Exceptions to Madelung’s Rule (Shown on Periodic Table)
Aufbau principle is a rule for building up the electronic configuration of atoms and molecules. It states that a maximum of two electrons are put into orbitals in the order of increasing orbital energy: the lowest-energy orbitals are filled before electrons are placed in higher-energy orbitals.
The order in which subshells are filled in atoms is given by the (n + ℓ) rule, also known as the Madelung rule (after Erwin Madelung), or the Janet rule or the Klechkowsky rule (after Charles Janet or Vsevolod Klechkovsky in some, mostly French and Russian-speaking, countries), or the diagonal rule. Orbitals with a lower n + ℓ value are filled before those with higher n + ℓ values. In this context, n represents the principal quantum number and ℓ the azimuthal quantum number; the values ℓ = 0, 1, 2, 3 correspond to the s, p, d, and f labels, respectively. The subshell ordering by this rule is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s, ...
The rule is based on the total number of nodes in the atomic orbital, n + ℓ, which is related to the energy. In the case of equal n + ℓ values, the orbital with a lower n value is filled first. The fact that most of the ground state configurations of neutral atoms fill orbitals following this n + ℓ, n pattern was obtained experimentally, by reference to the spectroscopic characteristics of the elements. However there are exceptions to Madelung’s energy ordering rule as represented in above image.
(for more See: https://en.wikipedia.org/wiki/Aufbau_principle)
|


